Scientific Perspective Consulting Inc.
Scientific insights through a strategic lens
Our services
From consulting on strategy development to practical implementation and scientific support for clinical trials, our comprehensive services can help your business thrive.
Clinical Strategy
Ensuring your clinical program is complete with the right types of studies to support regulatory approval. Recommendations on pilot studies, pivotal studies, biowaivers, and optimum partners for success.
Clinical Study Design
Establishing appropriate and robust clinical study design elements for protocols to ensure your product is tested robustly and can stand up to the rigor of regulatory scrutiny. This includes evaluation of sample size, sampling schedule, selection of endpoints, data and statistical analysis approach, and adherence to regulatory requirements.






Clinical Trial Results Interpretation
Evaluation of data results, discrepancies, reliability and perspective on next steps required for the research & development program.
Scientific Justifications
Whether you need to justify out of specification findings on the product's clinical impact, or provide a robust explanation to regulators to defend your application, we work collaboratively to strategize and review scientific justifications and highlight potential risks to the approval.
Scientific Review of Clinical Study Proposals
Obtain an independent perspective on suitability of CRO clinical study proposals, costs, and design elements such as study objectives, endpoints, sample size, sampling schedule, criteria for inclusion/exclusion, withdrawal criteria and adherence to regulatory requirements.






Scientific Review of Clinical Study Reports
Obtain an independent review of clinical study reports, with highlights of any deficiencies or discrepancies, adherence to protocol requirements, adherence to regulatory submission requirements, and highlight of risks with the study outcome or report to prepare in advance for potential deficiencies.
Biowaivers
Clinical trials are costly and time consuming, opportunities to pursue biowaivers can expedite product development and aid in affordable development. Assessment of suitability of biowaivers, and preparing BCS-based and Foreign reference biowaivers.
Regulatory Correspondence
Controlled correspondence with regulatory agencies to justify alternate approaches to product development, and influencing regulatory guidance provide a unique opportunity to gain valuable insight and stay ahead of competitors.
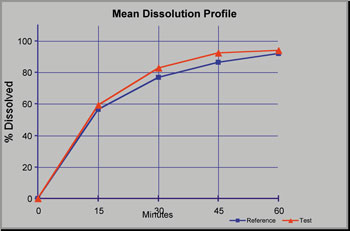
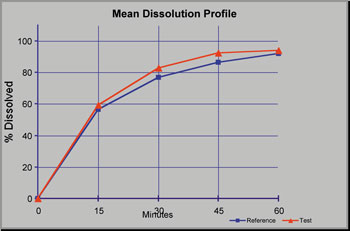




Good Clinical Practice (GCP) & Quality Assessments
Due diligence assessments of compliance with GCP ensures that contract research organizations (CROs) that provide clinical services are complying with guidelines. Sponsors are ultimately responsible for the study conduct and data generated by their contracted organizations. Quality risk mitigation aids the development program.
Competitive Intelligence Surveillance
Surveilling development programs of competitors and the industry is an important aspect to ensuring the proper portfolio targets, and being able to respond to changes in the marketplace in real-time, avoiding costly delays due to lack of information on industry developments. This requires regular and dedicated surveillance of a wide range of information to support decision making for management.
Standard Operating Procedure (SOP) Development and Review
Evaluating or writing SOPs in compliance with GCP / GLP, pertaining to procedures for: clinical trial oversight by sponsor, trial master file, study planning, clinical trial conduct, data analysis, report writing, quality audits, or other procedures.


Other Requests
If there is a service you are looking for that is not listed, please ask whether we can support your needs!
